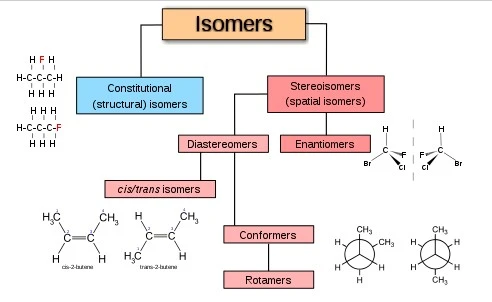
| The relationship between the two compounds is that they are both organic molecules. Both of these molecules contain carbon atoms, and they are both essential to life as we know it. 1. The first molecule, glucose, is a sugar molecule that is found in many foods. It is essential for the body to create energy. 2. The second molecule, cellulose, is a structural compound that helps plants to stand upright and provides support for their leaves and flowers. The relationship between the two compounds is that they are both organic compounds. They are both made up of carbon and hydrogen atoms. How Many Stereoisomers are Possible for a Compound With the Following Constitution? If a molecule has the constitution R-S-R’, that means it has three chiral centers (marked with an asterisk). This gives the molecule eight possible stereoisomers, as shown below. The first stereoisomer is called “RSR'”. The second is “SRR'”. The third is “RR’S”. The fourth is “R’RS”. The fifth is “RSSR'”. The sixth is “SR’S”. The seventh is “SSRR'”. And the eighth is “SRSR'”. What is a Constitutional Isomer in Organic Chemistry? A constitutional isomer is an organic compound that has the same molecular formula as another organic compound, but a different atomic arrangement. The different atomic arrangements can be due to the different ways that the atoms can be bonded together, or the different locations of functional groups within the molecule. Constitutional isomers often have very different chemical and physical properties from each other. One example of constitutional isomers is compounds that have the same molecular formula but a different carbon skeleton. For example, propane (C3H8) and butane (C4H10) are constitutional isomers. Both compounds have the molecular formula C3H8, but they have a different number of carbons in their skeletons. This means that they have different structures and properties. Propane is a gas at room temperature, while butane is a liquid. They also have different boiling points (-42 degrees Celsius for propane and 0 degrees Celsius for butane). Another example of constitutional isomers is stereoisomers. Stereoisomers are molecules that have the same molecular formula and connectivity but differ in the three-dimensional arrangement of their atoms in space. One type of stereoisomerism is enantiomerism, which occurs when two molecules are mirror images of each other (like your left and right hand). A classic example of enantiomerism are molecules known as optical isomers: these pairs of molecules rotate plane-polarized light by equal amounts but in opposite directions (+/- 50 degrees). Because they rotate plane-polarized light differently, they look like mirror images of each other under a polarizing microscope – even though they’re not actually physically mirror images! Which of the Following Statements About Constitutional Isomers is Not True? There are many different types of constitutional isomers, but not all of them are true. For example, one type of constitutional isomer is when two molecules have the same chemical formula but different arrangements of atoms. Another type of constitutional isomer is when two molecules have the same atoms but in different proportions. And yet another possibility is when two molecules have different numbers of atoms altogether. So, which of these statements about constitutional isomers is not true? The statement is that they all have the same chemical formula. How Many Stereogenic Centers are Present in the Following Compound? When it comes to stereogenic centers, or chiral centers, in molecules, each one can be thought of as a kind of “handedness”. Just like how our hands are either right-handed or left-handed, these stereogenic centers can be either R (right) or S (left). The number of stereogenic centers present in a molecule can give us clues about its possible handedness. In the case of the molecule shown above, there are four stereogenic centers present. This means that the molecule could be either RRRS or SSRR. However, because there are an equal number of R and S groups present, we know that this molecule is actually racemic. This term just means that the molecule has an equal mixture of both R and S enantiomers present. The two compounds are related in that they are both involved in the process of photosynthesis. Photosynthesis is the process by which plants use sunlight to convert carbon dioxide into organic matter, such as glucose. Chlorophyll is a pigment found in plants that helps them absorb light energy from the sun, while carotene is another pigment that helps protect plants from damage by absorbing ultraviolet light. |